
It also contributes to the hydration of organic compounds containing oxygen or nitrogen atoms and thus accounts for the much greater aqueous solubility of alcohols than hydrocarbons. Thus, hydrogen bonding is one of the principal mechanisms of hydration of anions in aqueous solution (the bonding of H 2O molecules to the solute species) and hence contributes to the ability of water to act as a good solvent for ionic compounds.
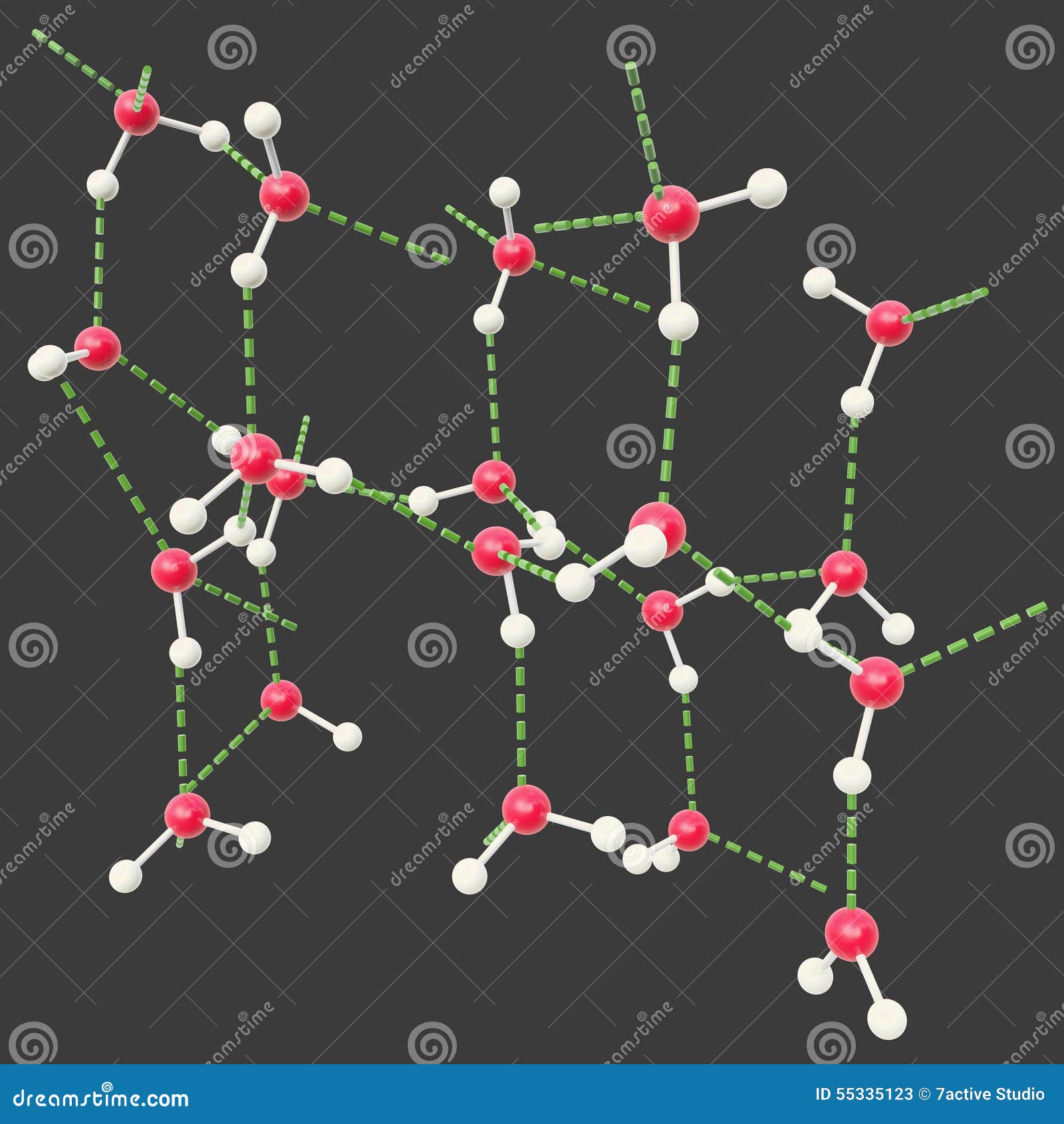
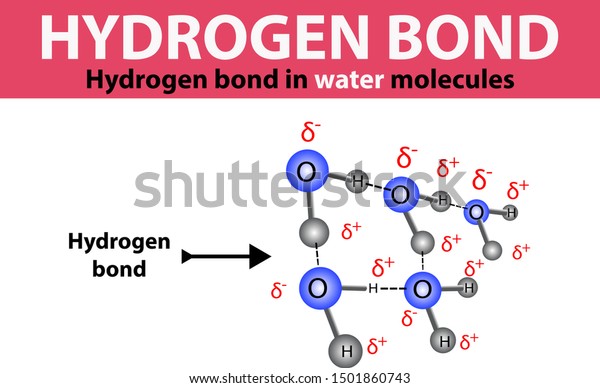
Hydrogen bonding occurs to atoms other than nitrogen, oxygen, and fluorine if they carry a negative charge and hence are rich in readily available electrons. The explanation lies in the small size of the hydrogen atom, which enables the balance of energies in the molecular orbital scheme to be favourable to bonding. Once again, on encountering the hydrogen bond, one encounters a twist in the conventional attitude the question raised by this interpretation is not why such a bond occurs but why it does not occur more generally. Hence, the net effect is to lower the energy of the AHB grouping and thus to constitute an intermolecular bond. They occupy the bonding and nonbonding orbitals, leaving the antibonding orbital vacant. There are four electrons to accommodate (two from the original A―H bond and two from the lone pair). When the three atoms are aligned, these three orbitals can form three molecular orbitals: one bonding, one largely nonbonding, and one antibonding. One that fits into the general scheme of this article is to think of the A―H unit as being composed of an A atomic orbital and a hydrogen 1 s orbital and to consider a lone pair of electrons on B as occupying a B orbital. Many interpretations of the hydrogen bond have been proposed. The hydrogen bond is also responsible for the existence as solids of many organic molecules containing hydroxyl groups (―OH) the sugars glucose and sucrose are examples. It is responsible, for example, for the existence of water as a liquid at normal temperatures because of its low molar mass, water would be expected to be a gas. A hydrogen bond is about 10 times as strong as the other interactions described above, and when present it dominates all other types of intermolecular interaction. ♻, where A and B are atoms of any of the three elements mentioned above and the hydrogen atom lies on a straight line between the nuclei of A and B.This interaction is the hydrogen bond, an interaction of the form A―H
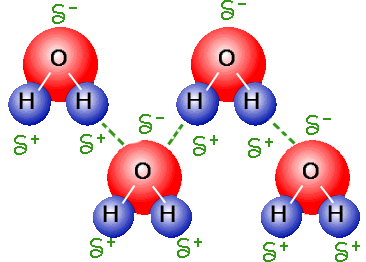
However, there is one important intermolecular interaction specific to molecules containing an oxygen, nitrogen, or fluorine atom that is attached to a hydrogen atom. The interactions described so far are not limited to molecules of any specific composition. SpaceNext50 Britannica presents SpaceNext50, From the race to the Moon to space stewardship, we explore a wide range of subjects that feed our curiosity about space!.Learn about the major environmental problems facing our planet and what can be done about them! Saving Earth Britannica Presents Earth’s To-Do List for the 21st Century.100 Women Britannica celebrates the centennial of the Nineteenth Amendment, highlighting suffragists and history-making politicians.
Hydrogen bond how to#
COVID-19 Portal While this global health crisis continues to evolve, it can be useful to look to past pandemics to better understand how to respond today.Student Portal Britannica is the ultimate student resource for key school subjects like history, government, literature, and more.This Time in History In these videos, find out what happened this month (or any month!) in history.#WTFact Videos In #WTFact Britannica shares some of the most bizarre facts we can find.Demystified Videos In Demystified, Britannica has all the answers to your burning questions.Britannica Classics Check out these retro videos from Encyclopedia Britannica’s archives.Britannica Explains In these videos, Britannica explains a variety of topics and answers frequently asked questions.


 0 kommentar(er)
0 kommentar(er)
